【Original Articles】
- Vicinal Difunctionalization of Alkenes Using Vinyl Triflates Leading to γ-Trifluoromethylated Ketones
Kawamoto, T.; Kawabata, T.; Noguhi, K.; Kamimura, A.
Org. Lett. 2022, 24, 324–327.
https://doi.org/10.1021/acs.orglett.1c03988

-
One-Pot Synthesis of CF3 ‑ Substituted Vinyl Trifluoromethanesulfonamides from Imines and Trifluoromethanesulfonic Anhydride
Kawamoto, T.; Ikawa, K.; Kamimura, A.
J. Org. Chem. 2021, 86, 15818–15824.
https://doi.org/10.1021/acs.joc.1c01969

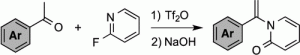
- Synthesis of 1-(1-Arylvinyl)Pyridin-2(1 H )-Ones from Ketones and 2-Fluoropyridine .
Kawamoto, T.; Ikeda, S.; Kamimura, A.
J. Org. Chem. 2021, 86, 13783–13789.
https://doi.org/10.1021/acs.joc.1c01615
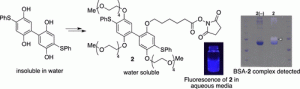
- Development of Water Solubility of 2-Phenylsulfanylhydroquinone Dimer Dye.
Kamimura, A.; Umemoto, H.; Kawamoto, T.; Honda, T.
ACS Omega 2021, 6, 9254–9262.
https://doi.org/10.1021/acsomega.1c00703
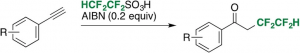
- Redox-neutral Tetrafluoroethylation of Aryl Alkynes with 1,1,2,2-Tetrafluoroethane sulfonic acid leading to α-Tetrafluoroethylated Acetophenones
Kawamoto, T.; Noguchi, K.; Sasaki, R.; Takata, R.; Matsubara, H.; Kamimura, A.
Chem. Eur. J. 2021, 9529–9534.
https://doi.org/10.1002/chem.202100137
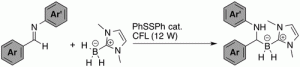
- Inverse Hydroboration of Imines with NHC-Boranes Is Promoted by Diphenyl Disulfide and Visible Light.
Kawamoto, T.; Morioka, T.; Noguchi, K.; Curran, D. P.; Kamimura, A.
Org. Lett. 2021, 23, 1825–1828.
https://doi.org/10.1021/acs.orglett.1c00230
-
Hydrodecyanation of Secondary Alkyl Nitriles and Malononitriles to Alkanes using DiMeImd-BH3.
Kawamoto, T.; Oritani, K.; Kawabata, A.; Morioka, T.; Matsubara, H.; Kamimura, A.
J. Org. Chem. 2020, 85, 6137.
http://dx.doi.org/10.1021/acs.joc.0c00105
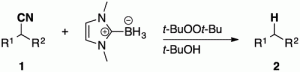
- Highly Cumulated Radical Cascade Reaction of aza-1,6-Enyenes: Stereoselective Synthesis of exo-Methylene Piperidines.
Kamimura, A.; Itaya, T.; Yoshinaga, T.; Nozawa, R.; Kawamoto, T.; Sumimoto, M.; Uno, H.
Eur. J. Org. Chem. 2020, 700.
http://dx.doi.org/10.1002/ejoc.202000034
- 2‐Sulfanylhydroquinone Dimer as a Switchable Fluorescent Dye.
Kamimura, A.; Sakamoto, S.; Umemoto, H.; Kawamoto, T.; Sumimoto, M.
Chem. Eur. J. 2019, 25, 14081.
http://dx.doi.org/10.1002/chem.201903436

Selected as Cover Feature
http://dx.doi.org/10.1002/chem.201904089

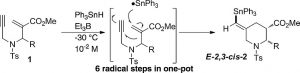
- The regioselective trifluoromethylation of 1,3-bis(vinyl triflates) in the absence of external trifluoromethyl sources.
Kawamoto, T.; Sasaki, R.; Kamimura, A.; Matsubara, H.
J. Fluorine Chem. 2019, 221, 66–69.
http://dx.doi.org/10.1016/j.jfluchem.2019.04.002
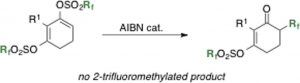
- Depolymerization of polyamide 6 in hydrophilic ionic liquids.
Kamimura, A.; Shiramatsu, Y.; Kawamoto, T.
Green Energy and Environment 2019, 4, 166-170.
http://dx.doi.org/10.1016/j.gee.2019.01.002
- Comparison of homofugality among alkyl groups attached to tin atom.
Kamimura, A.; Yoshinaga, T.; Miyazaki, K.; Kawamoto, T.
Heteroatom Chem. 2018, 2, e21469–7.
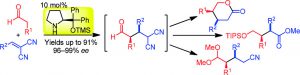
http://dx.doi.org/10.1002/hc.21469 - Hayashi, Y.; Kranidiotis-Hisatomi, N.; Sakamoto, D.; Oritani, K.; Kawamoto, T.; Kamimura, A.
Asymmetric Michael Reaction of Aldehydes and Dicyanoalkenes Catalyzed by Diphenylprolinol Silyl Ether.
Eur. J. Org. Chem. 2018, 6843–6847. http://dx.doi.org/10.1002/ejoc.201800831
- Development of a microwave-assisted sustainable conversion of furfural hydrazones to functionalised phthalimides in ionic liquids.
Karaluka, V.; Murata, K.; Masuda, S.; Shiramatsu, Y.; Kawamoto, T.; Hailes, H. C.; Sheppard, T. D.; Kamimura, A.
RSC Adv. 2018, 8, 22617–22624. http://dx.doi.org/10.1039/C8RA03895C

- Solubility-switchable ionic liquids: A control of hydrophilicity and hydrophobicity using a protective group.
Kamimura, A.; Shiramatsu, Y.; Murata, K.; Kawamoto, T.
Chem. Lett. 2018, 47, 1079–1081. http://dx.doi.org/10.1246/cl.180382

- Deltaarenes; novel macrocyclic molecules that are readily available from 1,4-benzoquinone and benzene dithiols.
Kamimura, A.; Watanabe, R.; Fukumitsu, T.; Ikeda, K.; Kawamoto, T.; Sumimoto, M.; Mori, S.; Uno, H.
Tetrahedron 2018, 74, 5303–5308. http://dx.doi.org/10.1016/j.tet.2018.04.070
- Thiol-Catalyzed Radical Decyanation of Aliphatic Nitriles with Sodium Borohydride
Kawamoto, T.; Oritani, K.; Curran, D. P.; Kamimura, A.
Org. Lett. 2018, 20, 2084–2087. https://dx.doi.org/10.1021/acs.orglett.8b00626

- Tris (trimethylsilyl) silane-Mediated Reductive Decyanation and Cyano Transfer Reactions of Malononitriles
Kawamoto, T.; Shimaya, Y.; Curran, D. P.; Kamimura, A.
Chem. Lett. 2018, 47, 573–575. https://doi.org/10.1246/cl.171231
- A kinetic study on the conversion of nylon 12 to methyl 12-hydroxydodecanoate in supercritical MeOH in the presence of carboxylic acid
Kamimura, A.; Ikeda, K.; Suzuki, S.; Kato, K.; Matsumoto, H.; Kaiso, K.; Yoshimoto, M.
Polym. Degr. Stabil. 2017, 146, 95–104. http://dx.doi.org/10.1016/j.polymdegradstab.2017.09.015
- An efficient and selective conversion of sorbitol in ionic liquids: Use of ion exchange resin as a solid acid catalyst
Kamimura, A.; Murata, K.; Kawamoto, T.
Tetrahedron Lett. 2017, 58, 3616–3618. http://dx.doi.org/10.1016/j.tetlet.2017.07.105

- Preparation of 2,3-trans-substituted piperidines from optically active β-amino-α-methylene esters: Synthesis of optically active (2S,3R)-(−)-epi-CP-99,994
Kamimura, A.; Yo, R.; Uno, H.
Tetrahedron 2017, 73, 4770–4778. http://dx.doi.org/10.1016/j.tet.2017.06.054

- Synthesis of α-Trifluoromethylated Ketones from Vinyl Triflates in the Absence of External Trifluoromethyl Sources
Kawamoto, T.; Sasaki, R.; Kamimura, A.
Angew. Chem. Int. Ed. 2017, 56, 1342–1345. http://dx.doi.org/10.1002/anie.201608591

- Efficient depolymerization and chemical conversion of polyamide 66 to 1,6-hexanediol
Matsumoto, H.; Akinari, Y.; Kaiso, K.; Kamimura, A.
J. Mater. Cycles Waste Manag. 2017, 19, 326–331. http://dx.doi.org/10.1007/s10163-015-0425-4
- Modulation of cellulase activity by charged lipid bilayers with different acyl chain properties for efficient hydrolysis of ionic liquid-pretreated cellulose
Mihono, K.; Ohtsu, T.; Ohtani, M.; Yoshimoto, M.; Kamimura, A.
Colloids Surface B 2016, 146, 198–203. http://dx.doi.org/10.1016/j.colsurfb.2016.06.005

- Asymmetric Synthesis of Bicyclic Nitrocyclopropanes from Primary Nitro Compounds and Stereoselective Formation of Tetrahydro-2H-cyclopenta[b]furans via Ring Expansion/Cyclization Reaction
Kamimura, A.; Moriyama, T.; Ito, Y.; Kawamoto, T.; Uno, H.
J. Org. Chem. 2016, 81, 4664–4681. http://dx.doi.org/10.1021/acs.joc.6b00566

- A radical cascade reaction triggered by thiyl radical; An approach toward synthesis of tricyclic structure of platensimycin
Kamimura, A.; Kawakami, Y.
Phosphous, Sulfur Silicon Relat. Elem. 2016, 191, 259–262. http://dx.doi.org/10.1080/10426507.2015.1064922

- A radical cascade reaction of aza-1,6-enyne compounds using allyltributyltin
Kamimura, A.; Miyazaki, K.; Kawamoto, T.; Uno, H.
Tetrahedron 2016, 72, 7722–7726. http://dx.doi.org/10.1016/j.tet.2016.04.078

- Oxidative synthesis of isoxazoline-N-oxide from optically active nitro alcohols
Moriyama, T.; Kawamoto, T.; Uno, H.; Kamimura, A.
Heterocycles 2016, 92, 1479–1489. http://dx.doi.org/10.3987/COM-16-13497

- An efficient conversion of lysine to 2-aminocaprolactam
Matsumoto, H.; Kaiso, K.; Kamimura, A.
Heterocycles 2016, 92, 337–345. http://dx.doi.org/10.3987/COM-15-13358

- Synthesis of 6-Arylpyridin-3-ols by Oxidative Rearrangement of (5-Arylfurfuryl)amines
Fürst, M.C.D.; Sauer, C.S.; Moriyama, T.; Kamimura, A.; Heinrich, M.R.
Eur. J. Org. Chem. 2016, 2016, 3051–3055. http://dx.doi.org/10.1002/ejoc.201600377

- An oxidative cyclopropanation reaction of primary nitro compounds using Fe2O3
Moriyama, T.; Ito, Y.; Koyama, Y.; Kawamoto, T.; Kamimura, A.
Tetrahedron Lett. 2016, 57, 3127–3128. http://dx.doi.org/10.1016/j.tetlet.2016.06.013

- A regioselective double Stille coupling reaction of bicyclic stannolanes
Kamimura, A.; Tanaka, T.; So, M.; Itaya, T.; Matsuda, K.; Kawamoto, T.
Org. Biomol. Chem. 2016, 14, 8109–8122. http://dx.doi.org/10.1039/c6ob01018k

- Synthesis of unsymmetrical 3,7-bisarylthio-2,8-dioxydibenzofuran and its physical properties
Kamimura, A.; Ishikawa, M.; Watanabe, R.; Sakamoto, S.; Uno, H.
Phosphous, Sulfur Silicon Relat. Elem. 2015, 190, 1219–1224. http://dx.doi.org/10.1080/10426507.2014.983598
- Synthesis of dibenzo[c,e][1,2]diazocines - A new group of eight-membered cyclic azo compounds
Nokubi, T.; Kindt, S.; Clark, T.; Kamimura, A.; Heinrich, M.R.
Tetrahedron Lett. 2015, 56, 316–320. http://dx.doi.org/10.1016/j.tetlet.2014.11.064

- A mechanistic study on the SHi reaction at tin atoms in a radical cascade reaction
Kamimura, A.; Yoshinaga, T.; Noguchi, F.; Miyazaki, K.; Uno, H.
Org. Chem. Front. 2015, 2, 713–720. http://dx.doi.org/10.1039/c5qo00063g

- Preparation of hydrophobic 2-phenylthiohydroquinone dimers and evaluation of their photophysical properties
Kamimura, A.; Takechi, Y.; Watanabe, R.
Heteroatom Chem. 2014, 25, 402–409. http://dx.doi.org/10.1002/hc.21169
- Preparation of 2,3-dihydrofurans via a double allylic substitution reaction of allylic nitro compounds
Nakano, T.; Miyazaki, K.; Kamimura, A.
J. Org. Chem. 2014, 79, 8103–8109. http://dx.doi.org/10.1021/jo5013042

- Gold(I)-catalyzed synthesis of optically active 1,4-oxazepan-7-ones
Kamimura, A.; Yamane, Y.; Yo, R.; Tanaka, T.; Uno, H.
J. Org. Chem. 2014, 79, 7696–7702. http://dx.doi.org/10.1021/jo501254x

- 不斉Aza-Morita-Baylis-Hillman等価反応を用いた複素環の合成
上村明男・石川慎吾
有機合成化学協会誌 2014, 72, 929–939. http://dx.doi.org/10.5059/yukigoseikyokaishi.72.929
- 4,4′-diarylsulfanyl-2,2′,5,5′-tetraoxybiaryl derivatives as a water-soluble fluorescent dye
Kamimura, A.; Nokubi, T.; Watanabe, R.; Ishikawa, M.; Nasu, K.; Uno, H.; Sumimoto, M.
J. Org. Chem. 2014, 79, 1068–1083. http://dx.doi.org/10.1021/jo402522y

- Rapid conversion of sorbitol to isosorbide in hydrophobic ionic liquids under microwave irradiation
Kamimura, A.; Murata, K.; Tanaka, Y.; Okagawa, T.; Matsumoto, H.; Kaiso, K.; Yoshimoto, M.
ChemSusChem 2014, 7, 3257–3259. http://dx.doi.org/10.1002/cssc.201402655

- Efficient Conversion of Polyamides to ω-Hydroxyalkanoic Acids: A New Method for Chemical Recycling of Waste Plastics
Kamimura, A.; Ikeda, K.; Suzuki, S.; Kato, K.; Akinari, Y.; Sugimoto, T.; Kashiwagi, K.; Kaiso, K.; Matsumoto, H.; Yoshimoto, M.
ChemSusChem 2014, 7, 2473–2477. http://dx.doi.org/10.1002/cssc.201402125

- A rapid route to aminocyclopropanes via carbamatoorganozinc carbenoids
Ishikawa, S.; Sheppard, T.D.; D'Oyley, J.M.; Kamimura, A.; Motherwell, W.B.
Angew. Chem. Int. Ed. 2013, 52, 10060–10063. http://dx.doi.org/10.1002/anie.201304720

- Hydrolysis of insoluble cellulose to glucose catalyzed by cellulase-containing liposomes in an aqueous solution of 1-butyl-3-methylimidazolium chloride
Yoshimoto, M.; Tanimura, K.; Tokunaga, K.; Kamimura, A.
Biosci. Biotech. Biochem. 2013, 29, 1190–1196. http://dx.doi.org/10.1002/btpr.1779
- A new water-soluble fluorescent dye based on 2-sulfanylhydroquinone dimers
Nokubi, T.; Nasu, K.; Watanabe, R.; Kamimura, A.
Chem. Lett. 2013, 42, 876–878. http://dx.doi.org/10.1246/cl.130348

- A radical cascade cyclization to prepare dihydrothiophenes induced by thiyl radicals as sulfur biradical equivalents
Kamimura, A.; Miyazaki, K.; Yamane, Y.; Yo, R.; Ishikawa, S.; Uno, H.; Sumimoto, M.
J. Org. Chem. 2013, 78, 7816–7822. http://dx.doi.org/10.1021/jo400975t

- Sub-stoichiometric oxidation of benzylic alcohols with commercially available activated MnO2under oxygen atmosphere: A green modification of the benzylic oxidation
Kamimura, A.; Komatsu, H.; Moriyama, T.; Nozaki, Y.
Tetrahedron 2013, 69, 5968–5972. http://dx.doi.org/10.1016/j.tet.2013.04.109

- Preparation of optically active bicyclodihydrosiloles by a radical cascade reaction
Miyazaki, K.; Yamane, Y.; Yo, R.; Uno, H.; Kamimura, A.
Beilstein J. Org. Chem. 2013, 9, 1326–1332. http://dx.doi.org/10.3762/bjoc.9.149

- Kinetic study of the 7- endo selective radical cyclization of N-tert-butyl-o-bromobenzylmethacryl amides: Kinetic investigation of the cyclization and 1,7-hydrogen transfer of aromatic radicals
Kamimura, A.; Kotake, T.; Ishihara, Y.; So, M.; Hayashi, T.
J. Org. Chem. 2013, 78, 3961–3971. http://dx.doi.org/10.1021/jo400326b

- Unexpected reductive double carbon-carbon bonds cleavage of bicyclic nitrocyclopropanes
Kamimura, A.; Ikeda, K.; Moriyama, T.; Uno, H.
Tetrahedron Lett. 2013, 54, 1842–1844. http://dx.doi.org/10.1016/j.tetlet.2013.01.094

- Preparation of optically active 4-aminopyrrolidines by radical addition/cyclization to chiral n-(2-(methoxyimino)ethyl)-β-amino-α- methylene esters
Kamimura, A.; Yamane, Y.; Uno, H.
Phosphous, Sulfur Silicon Relat. Elem. 2013, 188, 356–366. http://dx.doi.org/10.1080/10426507.2012.736893

- Pd-catalyzed tandem sp2-sp3 coupling reactions of chiral stannolanes: An efficient preparation of optically active tetrahydrobenz[f]isoindoles
Kamimura, A.; So, M.; Ishikawa, S.; Uno, H.
Org. Lett. 2013, 15, 1402–1405. http://dx.doi.org/10.1021/ol4003948

- Oxidation of benzyl alcohols by semi-stoichiometric amounts of cobalt-doped birnessite-type layered MnO2 under oxygen atmosphere
Kamimura, A.; Nozaki, Y.; Nishiyama, M.; Nakayama, M.
RSC Adv. 2013, 3, 468–472. http://dx.doi.org/10.1039/c2ra22117a

- Combination use of hydrophobic ionic liquids and LiCl as a good reaction system for the chemical conversion of cellulose to glucose
Kamimura, A.; Okagawa, T.; Oyama, N.; Otsuka, T.; Yoshimoto, M.
Green Chem. 2012, 14, 2816–2820. http://dx.doi.org/10.1039/c2gc35811e

- Nylon 6 depolymerization in supercritical alcohols studied by the QM/MC/FEP method
Kaweetirawatt, T.; Yamaguchi, T.; Hayashiyama, S.; Sumimoto, M.; Kamimura, A.; Hori, K.
RSC Adv. 2012, 2, 8402–8409. http://dx.doi.org/10.1039/c2ra20835k

- A study on the relationship between the twisted π-conjugate system of 1,5-diaryl-1,5-diazapenta-1,3-dienes and their photophysical properties
Kamimura, A.; Matsu, H.; Suzukawa, H.; Sato, E.; Sumimoto, M.; Uno, H.
Chem. Lett. 2012, 41, 984–986. http://dx.doi.org/10.1246/cl.2012.984

- Facile synthesis of quinone dimer derivatives substituted with sulfanyl groups and their properties
Kamimura, A.; Nokubi, T.; Nasu, K.; Takechi, Y.; Ishihara, Y.; Kato, K.; Noguchi, S.; Watanabe, M.; Shirai, M.; Sumimoto, M.; Uno, H.
Chem. Lett. 2012, 41, 950–951. http://dx.doi.org/10.1246/cl.2012.950

- Unexpected formation of stannolanes and trigonal bipyramidal tin complexes by radical cyclization reaction
Kamimura, A.; Ishikawa, S.; Noguchi, F.; Moriyama, T.; So, M.; Murafuji, T.; Uno, H.
Chem. Commun. 2012, 48, 6592–6594. http://dx.doi.org/10.1039/c2cc31753b

- Total synthesis of ent-calystegine B4 via nitro-Michael/aldol reaction
Kamimura, A.; Miyazaki, K.; Suzuki, S.; Ishikawa, S.; Uno, H.
Org. Biomol. Chem. 2012, 10, 4362–4366. http://dx.doi.org/10.1039/c2ob25386k

- Preparation of S RN1-type coupling adducts from aliphatic gem-dinitro compounds in ionic liquids
Kamimura, A.; Toyoshima, S.
Molecules 2012, 17, 4782–4790. http://dx.doi.org/10.3390/molecules17054782
- Concise synthesis of 2-benzazepine derivatives and their biological activity
So, M.; Kotake, T.; Matsuura, K.; Inui, M.; Kamimura, A.
J. Org. Chem. 2012, 77, 4017–4028. http://dx.doi.org/10.1021/jo300380z

- Heck-Matsuda reaction for allylic nitro compounds: A short asymmetric synthesis of an FTY720 derivative
Nakano, T.; Miyahara, M.; Itoh, T.; Kamimura, A.
Eur. J. Org. Chem. 2012, 2161–2166. http://dx.doi.org/10.1002/ejoc.201101703

- Stereoselective synthesis of 1-nitrobicyclo[3.1.0]hexanes and fused isoxazoline-N-oxides from primary nitro compounds
Kamimura, A.; Takeuchi, R.; Ikeda, K.; Moriyama, T.; Sumimoto, M.
J. Org. Chem. 2012, 77, 2236–2245. http://dx.doi.org/10.1021/jo202489v

- Direct conversion of polyamides to ω-hydroxyalkanoic acid derivatives by using supercritical MeOH
Kamimura, A.; Kaiso, K.; Suzuki, S.; Oishi, Y.; Ohara, Y.; Sugimoto, T.; Kashiwagi, K.; Yoshimoto, M.
Green Chem. 2011, 13, 2055–2061. http://dx.doi.org/10.1039/c1gc15172j

- Depolymerization of unsaturated polyesters and waste fiber-reinforced plastics by using ionic liquids: The use of microwaves to accelerate the reaction rate
Kamimura, A.; Yamamoto, S.; Yamada, K.
ChemSusChem 2011, 4, 644–649. http://dx.doi.org/10.1002/cssc.201000430

- Effective depolymerization of nylon-6 in wet supercritical hydrocarbons
Kaiso, K.; Sugimoto, T.; Kashiwagi, K.; Kamimura, A.
Chem. Lett. 2011, 40, 370–371. http://dx.doi.org/10.1246/cl.2011.370

- K-birnessite MnO2: A new selective oxidant for benzylic and allylic alcohols
Kamimura, A.; Nozaki, Y.; Ishikawa, S.; Inoue, R.; Nakayama, M.
Tetrahedron Lett. 2011, 52, 538–540. http://dx.doi.org/10.1016/j.tetlet.2010.11.114

- Chemo-Enzymatic Synthesis of a Multi-Useful Chiral Building. Block Molecule for the Synthesis of Medicinal Compounds
Nakano, T.; Yagi, Y.; Miyahara, M.; Kaminura, A.; Kawatsura, M.; Itoh, T.
Molecules 2011, 16, 6747–6757. https://doi.org/10.3390/molecules16086747 - Surfactant-induced electrodeposition of layered manganese oxide with large interlayer space for catalytic oxidation of phenol
Nakayama, M.; Shamoto, M.; Kamimura, A.
Chem. Mater. 2010, 22, 5887–5894. http://dx.doi.org/10.1021/cm101970b

- Improved preparation of recycled polymers in chemical recycling of fiber-reinforced plastics and molding of test product using recycled polymers
Yamada, K.; Tomonaga, F.; Kamimura, A.
J. Mater. Cycles Waste Manag. 2010, 12, 271–274. http://dx.doi.org/10.1007/s10163-010-0296-7
- Efficient chemical recycling of waste fiber-reinforced plastics: Use of reduced amounts of dimethylaminopyridine and activated charcoal for the purification of recovered monomer
Kamimura, A.; Akinari, Y.; Watanabe, T.; Yamada, K.; Tomonaga, F.
J. Mater. Cycles Waste Manag. 2010, 12, 93–97. http://dx.doi.org/10.1007/s10163-010-0276-y
- Asymmetric synthesis of 2-alkyl-substituted 2,5-dihydropyrroles from optically active aza-baylis-hillman adducts. Formal synthesis of (-)-trachelanthamidine
Ishikawa, S.; Noguchi, F.; Kamimura, A.
J. Org. Chem. 2010, 75, 3578–3586. http://dx.doi.org/10.1021/jo100315j

- Use of the diels-alder adduct of pyrrole in organic synthesis. Formal racemic synthesis of tamiflu
Kamimura, A.; Nakano, T.
J. Org. Chem. 2010, 75, 3133–3136. http://dx.doi.org/10.1021/jo1002856

- Intramolecular Pauson-Khand reaction of optically active aza-Baylis-Hillman adducts
Ishikawa, S.; Noguchi, F.; Uno, H.; Kamimura, A.
Tetrahedron Lett. 2010, 51, 2329–2331. http://dx.doi.org/10.1016/j.tetlet.2010.02.115

- Stereoselective synthesis of bicyclic nitrocyclopropanes by a radical-anion domino reaction
Kamimura, A.; Kadowaki, A.; Yoshida, T.; Takeuchi, R.; Uno, H.
Chem. Eur. J. 2009, 15, 10330–10334. http://dx.doi.org/10.1002/chem.200901920

- Preparation of novel functionalized ammonium salts that effectively catalyze depolymerization of Nylon-6 in ionic liquids
Yamamoto, S.; Kamimura, A.
Chem. Lett. 2009, 38, 1016–1017. http://dx.doi.org/10.1246/cl.2009.1016

- An efficient preparation of N-alkyl-2-benzazepine derivatives and investigation of their biological activity
Kamimura, A.; So, M.; Kuratani, T.; Matsuura, K.; Inui, M.
Bioorg. Med. Chem. Lett. 2009, 19, 3193–3195. http://dx.doi.org/10.1016/j.bmcl.2009.04.117

- Improved method for the formation of recycled resins from depolymerized products of waste fiber-reinforced plastics: Simple and effective purification of recovered monomers by washing with water
Kamimura, A.; Konno, E.; Yamamoto, S.; Watanabe, T.; Yamada, K.; Tomonaga, F.
J. Mater. Cycles Waste Manag. 2009, 11, 133–137. http://dx.doi.org/10.1007/s10163-008-0225-1
- Mechanistic study of 7-endo selective radical cyclization of the aryl radical
Kamimura, A.; Ishihara, Y.; So, M.; Hayashi, T.
Tetrahedron Lett. 2009, 50, 1727–1730. http://dx.doi.org/10.1016/j.tetlet.2009.01.132

- Formation of recycled plastics from depolymerized monomers derived from waste fiber-reinforced plastics
Kamimura, A.; Konno, E.; Yamamoto, S.; Watanabe, T.; Yamada, K.; Tomonaga, F.
J. Mater. Cycles Waste Manag. 2009, 11, 38–41. http://dx.doi.org/10.1007/s10163-008-0217-1
- A novel depolymerization of nylons in ionic liquids
Kamimura, A.; Yamamoto, S.
Polym. Adv. Technol. 2008, 19, 1391–1395. http://dx.doi.org/10.1002/pat.1199
- Stereoselective synthesis of azepines through the conjugate addition of formamides to nitroalkenes and subsequent intramolecular nitrile oxide cycloaddition reaction
Kamimura, A.; Yoshida, T.; Uno, H.
Tetrahedron 2008, 64, 11081–11085. http://dx.doi.org/10.1016/j.tet.2008.09.070

- DMAP as an effective catalyst to accelerate the solubilization of waste fiber-reinforced plastics
Kamimura, A.; Yamada, K.; Kuratani, T.; Oishi, Y.; Watanabe, T.; Yoshida, T.; Tomonaga, F.
ChemSusChem 2008, 1, 845–850. http://dx.doi.org/10.1002/cssc.200800151

- Supercritical secondary alcohols as useful media to convert polyamide into monomeric lactams
Kamimura, A.; Oishi, Y.; Kaiso, K.; Sugimoto, T.; Kashiwagi, K.
ChemSusChem 2008, 1, 82–84. http://dx.doi.org/10.1002/cssc.200700024

- 有機触媒と超臨界アルコールを用いたFRPの化学リサイクル
上村明男, 友永文昭, 山田和男
化学工業 2008, 59, 560–568. - 超臨界流体とイオン液体を使った新しいプラスチックの解重合反応―有機合成化学の新しい応用
上村明男
Chemical Times 2008, 208, 2–8. - Stereoselective conjugate addition of lactams to nitroalkenes and formal total synthesis of indolizidine 167B
Kamimura, A.; Nagata, Y.; Kadowaki, A.; Uchida, K.; Uno, H.
Tetrahedron 2007, 63, 11856–11861. http://dx.doi.org/10.1016/j.tet.2007.09.023

- An efficient method to depolymerize polyamide plastics: A new use of ionic liquids
Kamimura, A.; Yamamoto, S.
Org. Lett. 2007, 9, 2532–2535. http://dx.doi.org/10.1021/ol070886c

- Promotion of skin epithelial cell migration and wound healing by a 2-benzazepine derivative
Matsuura, K.; Kuratani, T.; Gondo, T.; Kamimura, A.; Inui, M.
Eur. J. Pharmacol. 2007, 563, 83–87. http://dx.doi.org/10.1016/j.ejphar.2007.02.014
- Asymmetric thio-Michael/nucleophilic addition domino reaction with chiral N-sulfinimines
Kamimura, A.; Okawa, H.; Morisaki, Y.; Ishikawa, S.; Uno, H.
J. Org. Chem. 2007, 72, 3569–3572. http://dx.doi.org/10.1021/jo062251h

- Stereoselective intramolecular 1,3-dipolar nitrile oxide cycloaddition reaction of N-formyl-β-nitroamides
Kadowaki, A.; Nagata, Y.; Uno, H.; Kamimura, A.
Tetrahedron Lett. 2007, 48, 1823–1825. http://dx.doi.org/10.1016/j.tetlet.2007.01.025

- Effective depolymerization waste FRPs by treatment with DMAP and supercritical alcohol
Kamimura, A.; Yamada, K.; Kuratani, T.; Taguchi, Y.; Tomonaga, F.
Chem. Lett. 2006, 35, 586–587. http://dx.doi.org/10.1246/cl.2006.586

- A convenient synthesis of medium-sized lactams through RCM reaction of oxyoxazolidinones
Kamimura, A.; Tanaka, K.; Hayashi, T.; Omata, Y.
Tetrahedron Lett. 2006, 47, 3625–3627. http://dx.doi.org/10.1016/j.tetlet.2006.03.152

- Formamide as an efficient nitrogen nucleophile for the Michael addition to nitroalkenes
Kamimura, A.; Kadowaki, A.; Nagata, Y.; Uno, H.
Tetrahedron Lett. 2006, 47, 2471–2473. http://dx.doi.org/10.1016/j.tetlet.2006.02.065

- Regioselective Michael addition of thiols to unsymmetrical fumaric diesters or esteramides
Kamimura, A.; Murakami, N.; Suzukawa, H.; Kawahara, F.
Phosphous, Sulfur Silicon Relat. Elem. 2005, 180, 1467–1468. http://dx.doi.org/10.1080/10426500590913113
- A practical method for chemical recycling of FRP. DMAP as an effective catalyst for degradation of FRPs in supercritical alcohol
Kamimura, A.; Kuratani, T.; Yamada K.; Tomonaga, F.
Feedstock recycling of Plastics, Selected Papers presented at the Third International Symposium on Feedstock Recycling of Plastics, Muller-Hagedorn, M.; Blckhorn, H. Eds. Universitatsverlag Karlsruhe, 2005, 525–529. - チオールの共役付加を用いた高選択的炭素骨格構築法の開発
上村明男
硫酸と工業, 2005, 58, 162–168. - Convenient switching of 7-endo/6-exo radical cyclization
Kamimura, A.; Taguchi, Y.
Tetrahedron Lett. 2004, 45, 2335–2337. http://dx.doi.org/10.1016/j.tetlet.2004.01.090

- 硫黄およびセレン求核剤を用いるマイケル付加反応の新展開
Kamimura, A.
有機合成化学協会誌 2004, 62, 705–715. http://dx.doi.org/10.5059/yukigoseikyokaishi.62.705
- A convenient stereoselective synthesis of β-lactams from β-hydroxy-α-thioalkylesters prepared from Michael/aldol tandem reaction or stereoselective addition of thiols to the Baylis-Hillman adducts
Kamimura, A.; Morita, R.; Matsuura, K.; Mitsudera, H.; Shirai, M.
Tetrahedron 2003, 59, 9931–9938. http://dx.doi.org/10.1016/j.tet.2003.10.035

- On the regioselectivity for the Michael addition of thiols to unsymmetrical fumaric derivatives
Kamimura, A.; Murakami, N.; Kawahara, F.; Yokota, K.; Omata, Y.; Matsuura, K.; Oishi, Y.; Morita, R.; Mitsudera, H.; Suzukawa, H.; Kakehi, A.; Shirai, M.; Okamoto, H.
Tetrahedron 2003, 59, 9537–9546. http://dx.doi.org/10.1016/j.tet.2003.10.007

- Oxyoxazolidinone as an auxiliary for heterocyclic synthesis. Enantioselective formation of N-unprotected 2-pyrrolidones from selenocarboxylate and allylamines via radical cyclization
Kamimura, A.; Omata, Y.; Tanaka, K.; Shirai, M.
Tetrahedron 2003, 59, 6291–6299. http://dx.doi.org/10.1016/S0040-4020(03)01015-9

- Convenient synthesis of 2-benzazepines via radical cyclization
Kamimura, A.; Taguchi, Y.; Omata, Y.; Hagihara, M.
J. Org. Chem. 2003, 68, 4996–4998. http://dx.doi.org/10.1021/jo030052h

- Magnesium cation-induced anti-aldol selective tandem Michael/aldol reaction
Kamimura, A.; Mitsudera, H.; Omata, Y.; Matsuura, K.; Shirai, M.; Kakehi, A.
Tetrahedron 2002, 58, 9817–9826. http://dx.doi.org/10.1016/S0040-4020(02)01299-1

- Enantioselective preparation of 3,4,5-trisubstituted 4,5-dihydroisoxazoles and their stereoselective elaboration of 5-side chain
Kamimura, A.; Kaneko, Y.; Ohta, A.; Matsuura, K.; Fujimoto, Y.; Kakehi, A.; Kanemasa, S.
Tetrahedron 2002, 58, 9613–9620. http://dx.doi.org/10.1016/S0040-4020(02)01255-3

- Stereoselective formation of optically active 2-oxy-1,3-oxazolidin-4-ones from chiral O-acylmandelamides or lactamides
Kamimura, A.; Omata, Y.; Kakehi, A.; Shirai, M.
Tetrahedron 2002, 58, 8763–8770. http://dx.doi.org/10.1016/S0040-4020(02)01057-8

- Regioselective Michael addition of thiols to tertiary fumaric amide esters
Kamimura, A.; Murakami, N.; Yokota, K.; Shirai, M.; Okamoto, H.
Tetrahedron Lett. 2002, 43, 7521–7523. http://dx.doi.org/10.1016/S0040-4039(02)01824-5

- Stereoselective formation of optically active 2-oxy-1,3-oxazolidin-4-ones and an efficient synthesis of optically active secondary 2-pyrrolidones
Omata, Y.; Kakehi, A.; Shirai, M.; Kamimura, A.
Tetrahedron Lett. 2002, 43, 6911–6914. http://dx.doi.org/10.1016/S0040-4039(02)01632-5

- Simple preparation of β-hydroxy-α-thiomethyl carbonyl compounds via stereoselective conjugate addition of thiol to Baylis-Hillman adducts
Kamimura, A.; Morita, R.; Matsuura, K.; Omata, Y.; Shirai, M.
Tetrahedron Lett. 2002, 43, 6189–6191. http://dx.doi.org/10.1016/S0040-4039(02)01318-7
- Stereoselective construction of multi-substituted tetrahydrofurans via three components-condensation reaction
Kamimura, A.; Mitsudera, H.; Matsuura, K.; Omata, Y.; Shirai, M.; Yokoyama, S.; Kakehi, A.
Tetrahedron 2002, 58, 2605–2611. http://dx.doi.org/10.1016/S0040-4020(02)00135-7

- Regioselective conjugate addition of thiols to unsymmetric fumaric esters in the presence of a lithium cation
Kamimura, A.; Kawahara, F.; Omata, Y.; Murakami, N.; Morita, R.; Otake, H.; Mitsudera, H.; Shirai, M.; Kakehi, A.
Tetrahedron Lett. 2001, 42, 8497–8500. http://dx.doi.org/10.1016/S0040-4039(01)01824-X

- Regioselective radical elimination of o-(bromoaryl)sulfides
Kamimura, A.; Mitsudera, H.
Tetrahedron Lett. 2001, 42, 7457–7460. http://dx.doi.org/10.1016/S0040-4039(01)01573-8

- A simple preparation of syn-NH-amide aldols and amide-Baylis-Hillman adducts via a Michael-aldol tandem process
Kamimura, A.; Omata, Y.; Mitsudera, H.; Kakehi, A.
J. Chem. Soc., Perkin Trans. 1 2000, 4499–4504. http://dx.doi.org/10.1039/b004721j
- An ab initio molecular orbital study on the magnesium controlled 1,3- cycloaddition of nitrile oxides and allylic alcohols with regio- and stereoselectivity
Fukuda, S.; Kamimura, A.; Kanemasa, S.; Hori, K.
Tetrahedron 2000, 56, 1637–1647. http://dx.doi.org/10.1016/S0040-4020(00)00042-9
- Synthesis of hydroximoyl chlorides from aldoximes and benzyltrimethylammonium tetrachloroiodate (BTMA ICl4)
Kanemasa, S.; Matsuda, H.; Kamimura, A.; Kakinami, T.
Tetrahedron 2000, 56, 1057–1064. http://dx.doi.org/10.1016/S0040-4020(99)01047-9
- Anti-Aldol selective tandem Michael/aldol reaction with magnesium selenolate and stereoselective preparation of tetrasubstituted tetrahydrofuran
Mitsudera, H.; Kakehi, A.; Kamimura, A.
Tetrahedron Lett. 1999, 40, 7389–7392. http://dx.doi.org/10.1016/S0040-4039(99)01516-6

- Stereoselective thio-Michael/aldol tandem reaction to α,β-unsaturated esters
Kamimura, A.; Mitsudera, H.; Asano, S.; Kidera, S.; Kakehi, A.
J. Org. Chem. 1999, 64, 6353–6360. http://dx.doi.org/10.1021/jo990548s

- Enantioselective preparation of 3,4,5-trisubstituted-4,5- dihydroisoxazoles and 4-substituted-5,6-dihydro-4H-[1,2]-oxazines by nitrile oxide cycloaddition to α-silyl allyl alcohols
Kamimura, A.; Kaneko, Y.; Ohta, A.; Kakehi, A.; Matsuda, H.; Kanemasa, S.
Tetrahedron Lett. 1999, 40, 4349–4352. http://dx.doi.org/10.1016/S0040-4039(99)00688-7

- Asymmetric Baylis-Hillman reactions: Catalysis using a chiral pyrrolizidine base
Barrett, A.G.M.; Cook, A.S.; Kamimura, A.
Chem. Commun. 1998, 2533–2534. http://dx.doi.org/
- A facile preparation of α-hydrazino-α,β-unsaturated ketones via aza- Baylis-Hillman reaction
Kamimura, A.; Gunjigake, Y.; Mitsudera, H.; Yokoyama, S.
Tetrahedron Lett. 1998, 39, 7323–7324. http://dx.doi.org/10.1016/S0040-4039(98)01570-6
- Stereoselective Michael/aldol tandem reaction triggered by thiolate anion or analogues
Kamimura, A.; Mitsudera, H.; Asano, S.; Kakehi, A.; Noguchi, M.
Chem. Commun. 1998, 1095–1096. http://dx.doi.org/
- Ab initio molecular orbital study on the mechanism of amide hydrolysis dependent on leaving groups
Hori, K.; Kamimura, A.; Ando, K.; Mizumura, M.; Ihara, Y.
Tetrahedron 1997, 53, 4317–4330. http://dx.doi.org/10.1016/S0040-4020(97)00158-0
- Molecular and crystal structure and properties of Te-containing p-terphenoquinone analogues
Tamura, R.; Takasuka, H.; Nagata, Y.; Azuma, N.; Matsumoto, A.; Sadaoka, Y.; Gunji, A.; Takahashi, K.; Kamimura, A.; Hori, K.
Mol. Cryst. Liq. Cryst. 1996, 278-279, 139–150. http://dx.doi.org/
- Catalytic asymmetric synthesis of α-methylene-β-hydroxy-ketones
Barrett, A.G.M.; Kamimura, A.
J. Chem. Soc., Chem. Commun. 1995, 1755–1756. http://dx.doi.org/10.1039/C39950001755
- Theoretical study on the mechanism of ester hydrolysis in micellar catalysis using model systems
Hori, K.; Kamimura, A.; Kimoto, J.; Gotoh, S.; Ihara, Y.
J. Chem. Soc., Perkin Trans. 2 1994, 2053–2058. http://dx.doi.org/
- First Successful Metal Coordination Control in 1, 3-Dipolar Cycloadditions. High-Rate Acceleration and Regio-and Stereocontrol of Nitrile Oxide Cycloadditions to the Magnesium Alkoxides of Allylic and Homoallylic Alcohols
Kanemasa, S.; Nishiuchi, M.; Kamimura, A.; Hori, K.
J. Am. Chem. Soc. 1994, 116, 2324–2339. http://dx.doi.org/10.1021/ja00085a012
- Preparation of Chiral Nitroxide Radicals and Spontaneous Optical Resolution by Recrystallization
Tamura, R.; Susuki, S.; Azuma, N.; Matsumoto, A.; Toda, F.; Kamimura, A.; Hori, K.
Angew. Chem. Int. Ed. 1994, 33, 878–879. http://dx.doi.org/10.1002/anie.199408781
- Reversing the regiochemical course of 1,3-dipolar cycloaddition of nitrile oxides by modificatin of dipolarophiles
Kamimura, A.; Hori, K.
Tetrahedron 1994, 50, 7969–7980. http://dx.doi.org/
- Reversing the Regiochemical Course of 1,3-Dipolar Cycloaddition of Nitrile oxides by Modification of Dipolarophiles
Kamimura, A.; Hori, K.
Tetrahedron 1994, 50, 7979–7980. http://dx.doi.org/10.1016/S0040-4020(01)85282-0
- An experimental and theoretical study on stereoselective addition to 3-formyl-Δ2-isoxazolines. Part 1. 1,3-anti-selectivity induced by BF3·OEt2
Kamimura, A.; Kakehi, A.; Hori, K.
Tetrahedron 1993, 49, 7637–7648. http://dx.doi.org/10.1016/S0040-4020(01)87239-2
- Theoretical study on crown compounds as building blocks of functional molecules I. The relation between the hole size and the number of atoms in the ring of cyclic ethers and amines
Hori, K.; Haruna, Y.; Kamimura, A.; Tsukube, H.; Inoue, T.
Tetrahedron 1993, 49, 3959–3970. http://dx.doi.org/10.1016/S0040-4020(01)89910-5
- Novel tellurium‐containing p‐terphenoquinone analogues: Preparation and unique redox properties of paramagnetic tellurium‐centered radical cation complexes
Tamura, R.; Nagata, Y.; Matsumoto, A.; Shimizu, H.; Ono, N.; Kamimura, A.; Hori, K.
Adv. Mater. 1993, 5, 719–721. http://dx.doi.org/10.1002/adma.19930051005
- Lewis Acid Promoted Stereoselective Carbon-Carbon Bond Formation of 3-Formyl-Δ2-isoxazolines
Kamimura, A.; Yoshihara, K.; Marumo, S.; Yamamoto, A.; Nishiguchi, T.; Hori, K.; Kakehi, A.
J. Org. Chem. 1992, 57, 5403–5413. http://dx.doi.org/10.1021/jo00046a023
- Stereoselective Synthesis of Cis 2,3-Disubstituted Cycloheptanones by Kinetic Protonation
Tamura, R.; Watabe, K.-I.; Kamimura, A.; Hori, K.; Yokomori, Y.
J. Org. Chem. 1992, 57, 4903–4905. http://dx.doi.org/10.1021/jo00044a027
- Synthesis of Photolabile Caged Amino Acids for Measuring Amino Acid Transporters
Aoshima, H.; Tanaka, D.; Kamimura, A.
Biosci. Biotech. Biochem. 1992, 56, 1086–1089. http://dx.doi.org/10.1271/bbb.56.1086
- Diastereoselective Cyclocondensation Reaction of 3-Formyl-2-isoxazolines
Kamimura, A.; Kakehi, A.
Chem. Lett. 1992, 1133–1136. https://doi.org/10.1246/cl.1992.1133 - 2−イソオキサゾリンの化学の最近の進歩―選択性の観点から
上村明男
有機合成化学協会誌 1992, 50, 808–825. - Displacement of Aliphatic Nitro Groups by Carbon and Heteroatom Nucleophiles
Tamura, R.;, Kamimura, A.; Ono, N.
Synthesis 1991, 423–434. http://dx.doi.org/10.1055/s-1991-26484 - syn- and anti-Selective preparation of 3-substltuted-Δ2-isoxazolines
Kamimura, A.; Marumo, S.
Tetrahedron Lett. 1990, 31, 5053–5056. http://dx.doi.org/10.1016/S0040-4039(00)97804-3
- Theoretical and Experimental Study on The Stereoselectivity of Michael Addition of Alkoxide Anion to Nitro Olefin
Hori, K.; Higuchi, S.; Kamimura, A.
J. Org. Chem. 1990, 55, 5900–5905. http://dx.doi.org/10.1021/jo00310a024
- Anti-Selective Michael Addition of Thiols and Their Analogues to Nitro Olefins
Kamimura, A.; Hori, K.; Sasatani, H.; Hashimoto, T.; Kawai, T.; Ono, N.
J. Org. Chem. 1990, 55, 2437–2442. http://dx.doi.org/10.1021/jo00295a036
- Ready Preparation of α-Nitroaldehyde Acetals from (E/Z)-1-Phenylthio-2-nitro-1-alkenes
Kamimura, A.; Nagashima, T.
Synthesis 1990, 694. http://dx.doi.org/10.1055/s-1990-26984 - Diastereoselective Ene Reaction of 3-Formyl-Δ2-isoxazolines
Kamimura, A.; Yamamoto, A.
Chem. Lett. 1990, 1991–1994. https://doi.org/10.1246/cl.1990.1991 - Lewis Acid Induced Nucleophilic Substitution Reaction of β-Nitro Sulfides
Kamimura, A.; Sasatani, H.; Hashimoto, T.; Ono, N.
J. Org. Chem. 1989, 54, 4998–5003. http://dx.doi.org/10.1021/jo00282a009
- Diastereoselective preparation of anti-β-amino alcohols via michael addition of alkoxide anions to nitroolefins and subsequent hydrogenation reaction
Kamimura, A.; Ono, N.
Tetrahedron Lett. 1989, 30, 731–734. http://dx.doi.org/10.1016/S0040-4039(01)80295-1
- A novel procedure for denitrohydrogenation by Na2S2O4-Et3SiH
Kamimura, A.; Kurata, K.; Ono, N.
Tetrahedron Lett. 1989, 30, 4819–4820. http://dx.doi.org/10.1016/S0040-4039(01)80517-7
- Regioselective Preparation of Cyclohexadienes or Aromatic Nitro Compounds by Diels-Alder Reactions of β-Sulfonylnitroolefins or β-Sulfinylnitroethylene
Ono, N.; Kamimura, A.; Kaji, A.
J. Org. Chem. 1988, 53, 251–258. http://dx.doi.org/10.1021/jo00237a005
- Stereoselective preparation of (E)-allyl alcohols via radical elimination from anti-γ-phenylthio-β-nitro alcohols
Kamimura, A.; Ono, N.
J. Chem. Soc., Chem. Commun. 1988, 1278–1280. http://dx.doi.org/10.1039/C39880001278
- A Facile Method for Preparation of Nitroenamines
Kamimura, A.; Ono, N.
Synthesis 1988, 921–922. http://dx.doi.org/10.1055/s-1988-27757 - On the Mechanism of Reductive Cleavage of the Carbon-Nitrogen Bond of Aliphatic Nitro Copmpounds with Tributyltin Hydride
Kamimura, A.; Ono, N.
Bull. Chem. Soc. Jpn. 1988, 61, 3629–3635. https://doi.org/10.1246/bcsj.61.3629 - A convenient procedure for the conversion of (E)-nitroalkenes to (Z)-nitroalkenes via erythro-β-nitroselenides
Ono, N.; Kamimura, A.; Kawai, T.; Kaji, A.
J. Chem. Soc., Chem. Commun. 1987, 1550–1551. http://dx.doi.org/10.1039/C39870001550
- Regioselective Diels-Alder reactions. The nitro group as a regiochemical control element
Ono, N.; Miyake, H.; Kamimura, A.; Kaji, A.
J. Chem. Soc., Perkin Trans. 1 1987, 1929–1935. http://dx.doi.org/
- Stereospecific Anti Radical Elimination Reaction from β-Nitro Sulfones
Ono, N.; Kamimura, A.; Kaji, A.
J. Org. Chem. 1987, 52, 5111–5116. http://dx.doi.org/10.1021/jo00232a010
- Lewis Acid Induced Nucleophilic Substitution Reactions of β-nitro Sulfides Via Episulfonium Ions
Ono, N.; Kamimura, A.; Sasatani, H.; Kaji, A.
J. Org. Chem. 1987, 52, 4133–4135. http://dx.doi.org/10.1021/jo00227a039
- β–ニトロスルフィドの還元脱離反応によるオレフィン合成
小野昇, 上村明男, 川合修次, 加治有恒
日本化学会誌 1987, 1338–1345. - Reaction of Carbanions Derived from α,β-Unsaturated Nitro Compounds with Electrophiles to Give α-Substituted Products
Ono, N.; Hamamoto, I.; Kamimura, A.; Kaji, A.; and Tamura, R.
Synthesis 1987, 258–260. http://dx.doi.org/10.1055/s-1987-27907 - β-Sulfonylnitroolefins as very reactive alkyne-equivalents in Diels-Alder reactions
Ono, N.; Kamimura, A.; Kaji, A.
Tetrahedron Lett. 1986, 27, 1595–1598. http://dx.doi.org/10.1016/S0040-4039(00)84323-3
- Lewis acid-induced nucleophilic substitution reactions of aliphatic nitro compounds with carbon nucleophiles
Ono, N.; Yanai, T.; Kamimura, A.; Kaji, A.
J. Chem. Soc., Chem. Commun. 1986, 1285–1287. http://dx.doi.org/10.1039/C39860001285
- Preparation of a New Type of Electron-Deficient Olefins: β-Phenylthio Nitro Olefins, β-Sulfinyl Nitro Olefins, and β-Sulfonyl Nitro Olefins
Ono, N.; Kamimura, A.; Kaji, A.
J. Org. Chem. 1986, 51, 2139–2142. http://dx.doi.org/10.1021/jo00361a044
- Regioselective Removal of Allylic Nitro Groups via Hydride Transfer
One, N.; Hamamoto, I.; Kamimura, A.; Kaji, A.
J. Org. Chem. 1986, 51, 3734–3736. http://dx.doi.org/10.1021/jo00369a043
- New Synthetic Methods. Conjugate Addition of Alkyl Groups to Electron Deficient Olefins with Nitroalkanes as Alkyl Anion Equivalents
Ono, N.; Kamimura, A.; Miyake, H.; Hamamoto, I.; Kaji, A.
J. Org. Chem. 1985, 50, 3692–3698. http://dx.doi.org/10.1021/jo00220a003
- Nitro Compounds as Nucleophilic Alkylidene Transfer Reagents
Ono, N.; Yanai, T.; Hamamoto, I.; Kamimura, A.; Kaji, A.
J. Org. Chem. 1985, 50, 2806–2807. http://dx.doi.org/10.1021/jo00215a052
- Denitrohydrogenation of aliphatic nitro compounds and a new use of aliphatic nitro compounds as radical precursors
Ono, N.; Miyake, H.; Kamimura, A.; Hamamoto, I.; Tamura, R.; Kaji, A.
Tetrahedron 1985, 41, 4013–4023. http://dx.doi.org/10.1016/S0040-4020(01)97180-7
- Michael Addition of Secondary Nitroalkanes to β-Substituted α,β-Unsaturated Compounds.
Ono, N.; Kamimura, A.; Kaji, A.;
Synthesis 1984, 226–228. http://dx.doi.org/10.1055/s-1984-30780 - A new synthesis of allylic alcohols or their derivatives via reductive elimination from γ-phenylthio-β-nitroalcohols with tributyltinhydride
Ono, N.; Kamimura, A.; Kaji, A.
Tetrahedron Lett. 1984, 25, 5319–5322. http://dx.doi.org/10.1016/S0040-4039(01)81593-8
- Conjugate addition of alkyl groups to α,β-unsaturated sulfoxides via Michael addition of nitroparaffins and subsequent denitration with tributyltin hydride
Ono, N.; Miyake, H.; Kamimura, A.; Tsukui, N.; Kaji, A.
Tetrahedron Lett. 1982, 23, 2957–2960. http://dx.doi.org/10.1016/S0040-4039(00)87505-X
【books】
- [2 + 1]-type cyclopropanation reactions, in Methods and applications of cycloaddition reactions in organic syntheses, Ed. by Nagatoshi Nishiwaki, John Wiley and Sons, New Jersey, 2014 (pp 1 – 66).
- 「化学は楽しいワンダーランド」単著 (丸善)
- 「実験化学講座 15巻 有機化合物の合成」分担執筆(丸善)
- 「有機合成のナビゲーター」単著 (丸善)
- 「初めての有機スペクトル解析」築部浩・宇野英満編 分担執筆(丸善)
- 「研究室で役立つ有機実験のナビゲーター」訳著(丸善)
- 「有機化学」奥山格著(丸善) 分担執筆
- 「研究室ですぐに使える有機合成のレシピ」訳著(丸善)
- 「各種手法による有機物の分解技術」分担執筆(情報機構)
- 「イオン液体IIIーナノ・バイオサイエンスへの挑戦ー」大野弘幸監修 分担執筆(シーエムシー出版)
- 「最先端材料システムOne Point2 イオン液体」高分子学会編集 分担執筆(共立出版)
- 「イオン液体の科学-新世代液体への挑戦-」イオン液体研究会 監督 西川恵子・大内幸雄・伊藤敏幸・大野弘幸・渡邊正義 編 分担執筆(丸善出版)
- 「新しい溶媒を用いた有機合成」分担執筆(S&T出版)
- 「フローチャートで考える有機反応機構」訳著(丸善出版)

