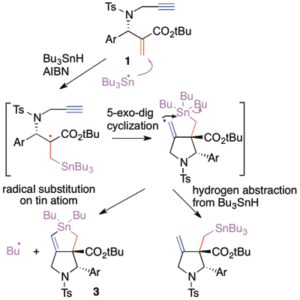
Radical chemistry using tin atom has been well-known chemistry. However we found a new aspect of tin chemistry under radical conditions. For example, a direct radical substitution reaction progresses if the molecular geometry is suitable. Very interesting radical cascade process containing, addition, rearrangement, and substitution reaction progresses. Recently, we successfully estimated a kinetic parameter of direct radical substitution reaction at a tin atom. Radical rearrangement including radical substitution process provided a new synthetic method of aza-heterocyclic compounds. Stannolane is a useful material for the double Stille coupling reaction to give tetrahydrobenz[f]isoindole derivatives. These novel aspects of tin chemistry will open a new methodology in organic synthesis.
Kamimura, A.; Miyazaki, K.; Kawamoto, T.; Uno, H.
Tetrahedron 2016, 72, 7722 – 7726: DOI: 10.1016/j.tet.2016.04.078 Kamimura, A.; Yoshinaga, T.; Noguchi, F.; Miyazaki, K.; Uno, H.
Org. Chem. Front. 2015, 2, 713-720.
Kamimura, A.; So, M.; Ishikawa, S.; Uno, H.
Org. Lett. 2013, 15, 1402 – 1405: DOI: 10.1021/ol4003948
Kamimura, A.; Ishikawa, S.; Noguchi, F.; Moriyama, T.; So, M.; Murafuji, T.; Uno, H.
Chem. Commun. 2012, 42, 6592-6594

