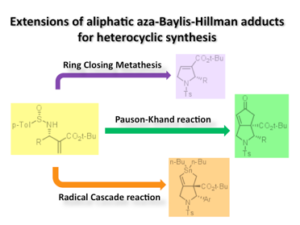
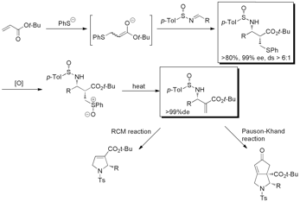
Asymmetric aza-Morita-Baylis-Hillman reaction is developed. The Michael/aldol domino reaction of chiral sulfinimines triggered by magnesium thiolate gave b-amino-a-phenylthiomethylesters in good yields. The enantioselectivity at the carbon adjacent to the amino group exceeds 90%, usually 99%. The following thermal elimination of sulfoxide afforded single enantiomer of chiral aza-Morita-Baylis-Hillman adducts in good yield. Use of the adducts provided a useful methods for heterocyclic synthesis and we have successfully achieved the formal synthesis of (-)-trachelanthamidine. An highly efficient SHi radical substitution reaction on silicon and sulfur atom was observed. We have recently developed a new synthetic method to prepare 7-membered ring using gold(I) catalyst and the 1,6-enyne compounds derived from chiral aza-Morita-Baylis-Hillman adducts
Publications
上村明男・石川慎吾, 有合化, 2014, 72, 929 – 939
Kamimura, A.; Yamane, Y.; Yo, R.; Tanaka, T.; Uno, H.
J. Org. Chem. 2014, 79, 7696 – 7702: DOI: 10.1021/jo501254x
Kamimura, A.; So, M.; Ishikawa, S.; Uno, H. Org. Lett. 2013, 15, 1402 – 1405: DOI: 10.1021/ol4003948
Miyazaki, K.; Yamane, Y.; Yo, R.; Uno, H.; Kamimura, A.
Beilstein J. Org. Chem. 2013, 9, 1326 – 1332; doi:10.3762/bjoc.9.149.
Kamimura, A.; Miyazaki, K.; Yamane, Y.; Yo, R.; Ishikawa, S.;
Uno, H.; Sumimoto, M. J. Org. Chem. 2013, 78, 7816 – 7822: DOI: 10.1021/jo400975t.
Kamimura, A.; Ishikawa, S.; Noguchi, F.; Moriyama, T.; So, M.; Murafuji, T.; Uno, H. Chem. Commun. 2012, 6592-6594
Ishikawa, S.; Noguchi, F.; Uno, H.; Kamimura, A. Tetrahedoron Lett. 2010, 51, 2329-2331
Ishikawa, S.; Noguchi, F.; Kamimura, A. J. Org. Chem. 2010, 75, 3578-3586
Kamimura, A.; Okawa, H.; Morisaki, Y.; Ishikawa, S.; Uno, H. J. Org. Chem. 2007, 72, 3569-3572

